
Methodology

Traditionally, the discovery of new antibacterial therapies has focused on finding new compounds for novel targets. However, this strategy is extremely expensive and time-consuming, hence it is not a practical solution to the urgent need for identification of alternative treatments for treponemal diseases. Repurposing strategies (i.e., finding new applications for existing drugs) can reduce the cost of drug development.
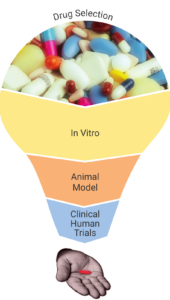
The main goal of the Trep-AB project is to repurpose an antibiotic that, delivered as an oral short-course or single dose to patients with syphilis or yaws, can cure the diseases. To that end, the project will proceed in a two-step process of analysis and validation:
- In vitro / in vivo animal model:We will use innovative technology consisting of a tissue-culture system very recently developed, which promotes the in vitro multiplication of treponemes, as a step prior to the necessary in vivo testing in animals. Only the components that are effective in vitro are to be tested in the rabbit model of syphilis, which is currently the most trusted method for testing T.p. susceptibility to antimicrobial agents.
- Clinical human trials:After the preclinical studies inform on which are the most promising investigational antibiotics, we will perform a randomized clinical trial, the best approach to test whether the selected antimicrobial agents are as efficacious as standard therapy to cure treponemal infections.
Also, we will use techniques that allow us to identify whether repeated episodes of syphilis/yaws are due to reinfection or to relapse. Syphilis/yaws recurrent infections provide the opportunity to perform comparative genomics to identify mutations that might be linked to a penicillin resistance phenotype.
The discovery of new effective antibiotics has the potential to change treatment policies worldwide.


